Watch our webinar to learn how Ansys Mechanical is changing the game for implant manufacturing companies like Mecuris: Modeling & Designing Patient-Specific Implants for Activities of Daily Living
How can medical implant manufacturers create highly customized and reliable devices that cater to individual patient needs?
Mecuris, a pioneer in virtual medical certification and individualized prostheses, leverages Ansys Mechanical's advanced simulation capabilities to overcome limitations in traditional implant design. This blog post will explore how Mecuris used Ansys Mechanical to revolutionize their implant design and development process (Download Case Study), ensuring better patient outcomes and faster regulatory approval.
I. Utilizing Precision Engineering for Patient-Specific Implants
Understanding the Individuality of Patients
As human biomechanics vary from one individual to another, Mecuris aims to provide each patient with a customized product whose quality is assured through simulation based on performance and safety evaluation. According to Franziska Glas, Quality Assurance Expert Lead (Simulation & Testing) at Mecuris GmbH, "Virtual test stands using Ansys Mechanical and Ansys optiSLang not only enable engineers to test a large set of design variants for medical certification, but also allow them to create a functional product from the first virtual design until the patient fitting of the final product."
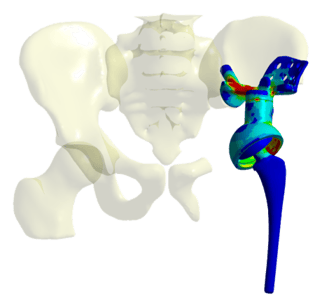
Leveraging Advanced Simulation Models
Ansys Mechanical enabled Mecuris to predict mechanical responses of complex deformable structures by considering known boundary conditions representing human motion or ISO standards. These advanced simulation models allowed Mecuris to account for each patient's unique biomechanics, resulting in highly tailored implant designs.
Iterative Design Studies for Optimal Results
Ansys Mechanical's precision engineering approach allowed Mecuris to create a design study with multiple geometric variants, providing a comprehensive understanding of how different design parameters could affect the implant's safety and performance. Through iterative design studies, Mecuris investigated the sensitivity of geometric or material parameters and optimized the implant design accordingly.
Achieving Patient-Specific, Best-Design Implants
This process culminated in the development of patient-specific, best-design implants that met safety and performance objectives. These highly customized implants not only provided enhanced safety and effectiveness but also improved patient satisfaction by addressing each individual's unique biomechanical requirements.
II. Speeding Up Design and Compliance in Implant Manufacturing
Facilitating Regulatory Compliance
The emerging field of virtual medical certification requires accurate and reliable FE-simulations to speed up the regulatory approval process of individualized prostheses and orthoses. Ansys Mechanical's powerful simulation capabilities enabled Mecuris to create highly customized implants while streamlining this approval process, ensuring products met and exceeded industry safety and effectiveness standards.
Reducing Testing Costs and Time
Traditional implant design approaches often involve the creation of multiple physical prototypes, which can be time-consuming and expensive. As Franziska Glas states, "In orthopedics, performing evaluations on test stands or human users is time-consuming and involves high costs. Only a limited number of designs can be manufactured and tested." Ansys Mechanical's advanced simulation models allowed Mecuris to significantly reduce testing costs and minimize the need for multiple prototypes, test stands, or renting testing facilities.
Related Case Study: HuMotech Enables Real-Time Testing and Assessment of Prosthetic Designs
Swift Adaptation to Patient Requirements
Ansys Mechanical facilitates a streamlined approach to design, development, and compliance, enabling manufacturers like Mecuris to quickly iterate on designs and respond to patient needs. This accelerated design process ensures the best possible outcomes for each individual.
Embracing Future-Proof Solutions for Enhanced Patient Outcomes
Improving Patient Safety and Satisfaction
By incorporating Ansys Mechanical's safety and performance parameter simulations into their digital orthopedic workshop, Mecuris offers certified prosthetist orthotists (CPOs) a streamlined and efficient method for individualized product design and 3D printing. This innovative approach reduces the time and resources required for traditional manufacturing methods, ultimately improving patient safety and satisfaction.
Adapting to Evolving Industry Trends
Ansys Mechanical equips companies like Mecuris to anticipate and respond to evolving industry trends and standards. With the ability to quickly adapt to new technologies, materials, and regulatory requirements, manufacturers can stay ahead of the curve and maintain a competitive edge in a rapidly changing market.
Reducing Development Costs and Increasing Efficiency
The adoption of Ansys Mechanical's advanced simulation capabilities and digital solutions not only results in improved patient outcomes but also leads to reduced costs and increased efficiency. By minimizing the need for physical prototypes, companies can save on materials and labor while accelerating the design process. Additionally, the integration of safety and performance parameter simulations ensures that products are optimized for both regulatory compliance and patient satisfaction, further reducing the risk of costly revisions or recalls.
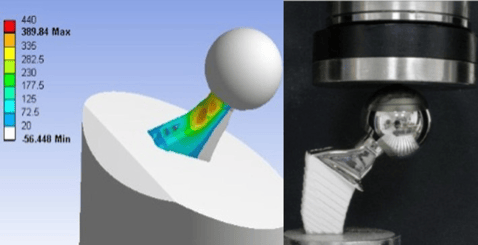
Ansys Mechanical offers a powerful, comprehensive solution for medical implant manufacturers seeking to create highly customized, safe, and effective devices.
Through advanced simulation capabilities, accelerated design processes, and future-proof solutions, companies like Mecuris can address critical pain points, meet regulatory requirements, and stay competitive in a rapidly evolving industry. By choosing Ansys Mechanical, manufacturers can focus on delivering exceptional patient experiences while navigating the regulatory landscape with ease.
See firsthand how Ansys Mechanical is changing the game for implant manufacturing companies like Mecuris at our upcoming webinar:
Modeling & Designing Patient-Specific Implants for Activities of Daily Living
Wednesday, May 24th at 12pm PST
Learn how you can revolutionize implant design, reduce development costs and get your product to market faster. Click here to register now.
May 15, 2023 12:37:08 PM

%202.png?width=1920&height=828&name=May%20Webinar%20(1920%20%C3%97%201080%20px)%202.png)